STEMCELL Technologies ImmunoCult ImmunoCult Human B Cell Expansion Kit
- 研究用
ImmunoCult™ Human B Cell Expansion Kit(ST-100-0645)は、ヒトのB細胞を活性化、増殖、および形質細胞に成熟させる培地キットです。血清、特殊な培養器具、あるいは煩雑なフィーダー細胞を使用せずに、わずか1週間以内の培養でB細胞を回収し、すぐに下流のアプリケーションに用いることができます。
キット構成品のImmunoCult™-XF B Cell Base Medium(ST-100-0646)とImmunoCult™-ACF Human B Cell Expansion Supplement(ST-10974)は、それぞれ個別に購入することもできます。
本品は、上流および下流の実験で用いる多くの製品と組み合わせて利用できます。例えば、EasySep™細胞分離キットでpan-B細胞、メモリーB細胞、ナイーブB細胞などの様々なB細胞サブセットを単離した後、すぐに本品で増殖することができます。
製品の特長
ImmunoCult™ Human B Cell Expansion Kitで、B細胞を無血清条件下で効率よく増殖
- 血清、フィーダー細胞、特殊な培養プレートを使用せずに、ヒトB細胞をin vitroで確実に増殖
- 無血清培地と、動物性成分不使用のサプリメントを含有
データ紹介
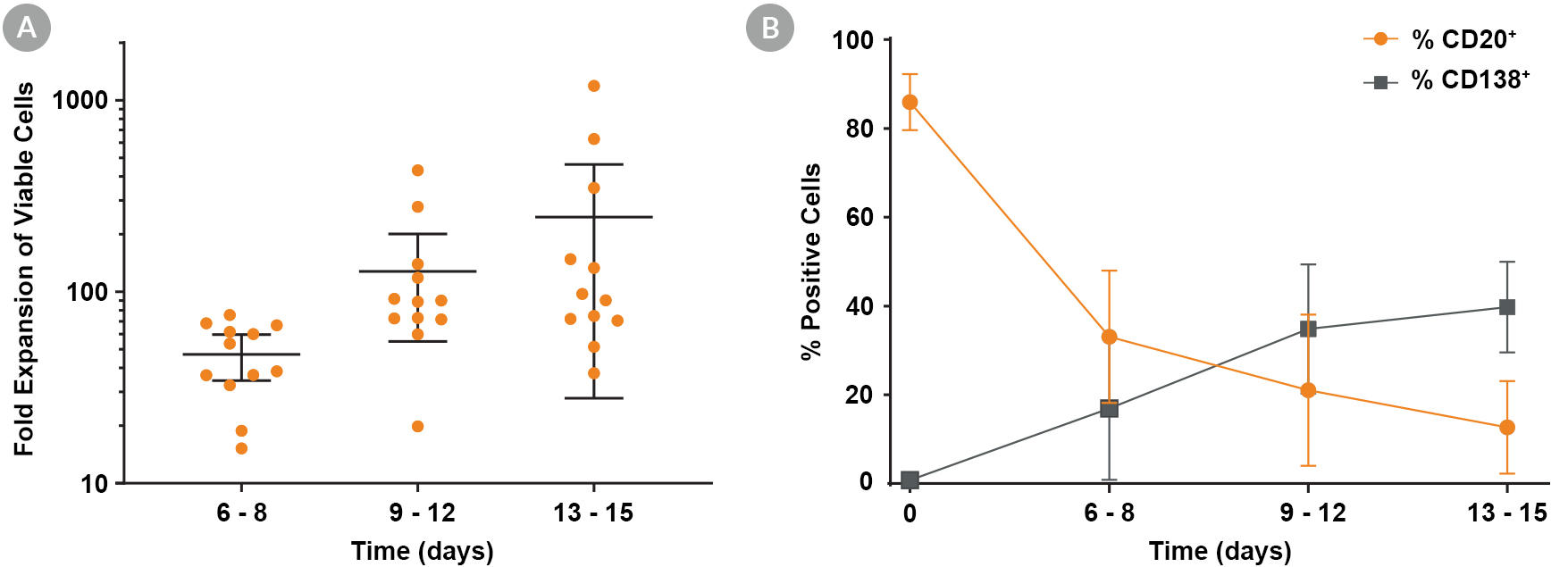
Figure 1. Expansion and Maturation of Human B Cells with ImmunoCult™-ACF Human B Cell Expansion Supplement
B cells isolated from human peripheral blood mononuclear cells (PBMCs) (leukopak) using EasySep™ Human Pan-B Cell Enrichment Kit (ST-19554) were seeded at 1 x 10^5 cells/well in 24-well tissue culture plates with ImmunoCult™-ACF Human B Cell Expansion Supplement and ImmunoCult™-XF B Cell Base Medium included in the ImmunoCult™ Human B Cell Expansion Kit. The cells were passaged every 3 - 4 days. (A) Fold expansion of viable cells is shown for n = 12 donors, with bars representing the mean and 95% confidence level (range 38- to 1190-fold at day 14 ± 1 day). (B) Expression of CD138 and CD20 was analyzed by flow cytometry at each timepoint (data represent % positive viable cells; mean ± 1 SD). The observed changes indicate maturation of B cells to plasma cells/blasts.
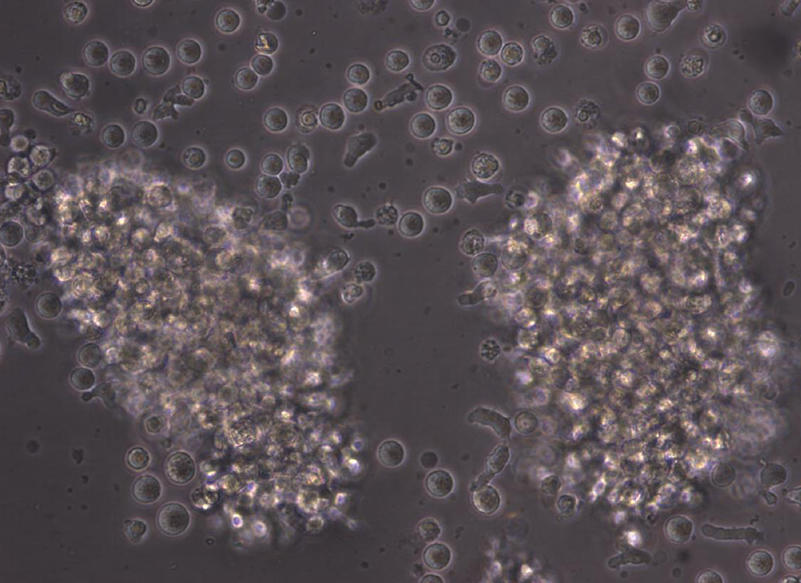
Figure 2. Light Microscopy Image of Cultured Human B Cells
B cells isolated from human PBMCs (leukopak) using EasySep™ Human Pan-B Cell Enrichment Kit were seeded at 1 x 10^5 cells/well in a 24-well tissue culture plate and cultured with the ImmunoCult™ Human B Cell Expansion Kit. The cells were passaged on day 4 after seeding and imaged at 40X magnification on day 6.
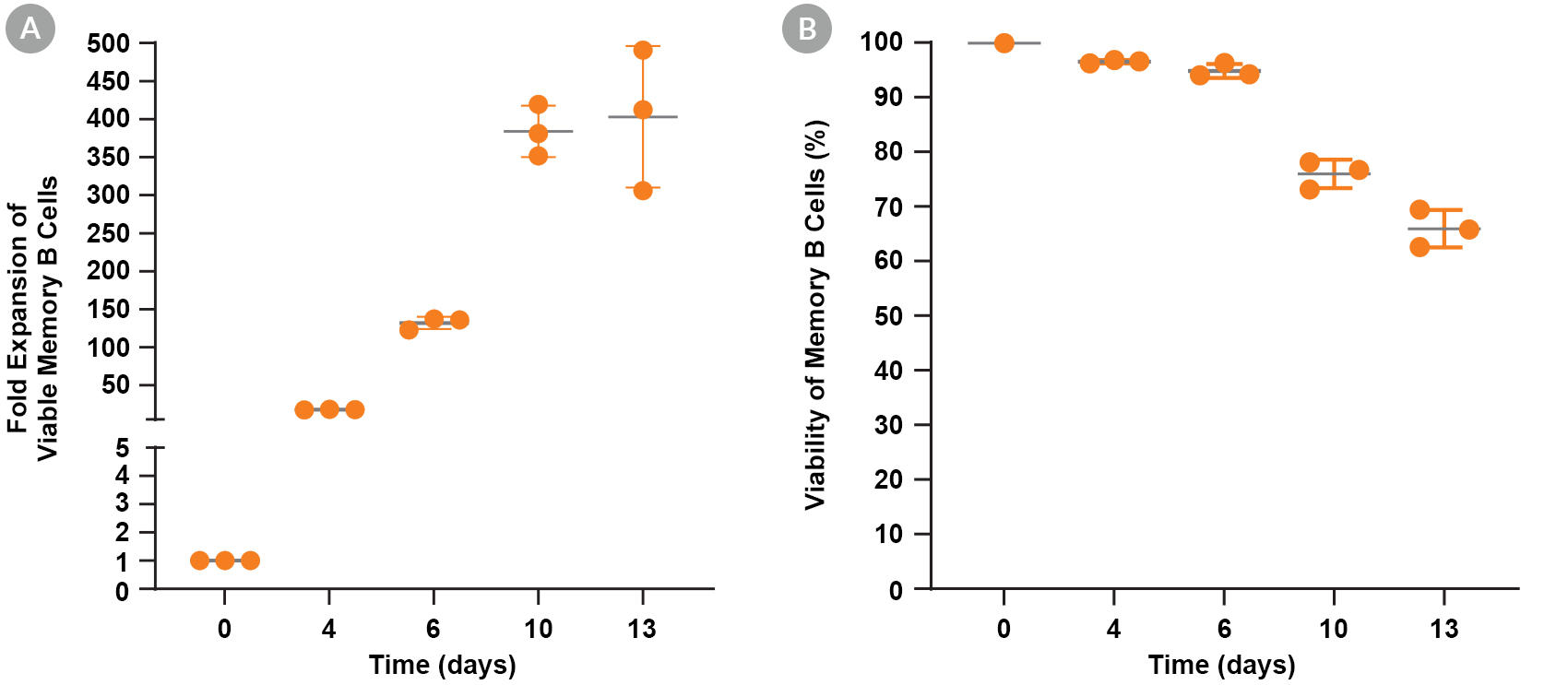
Figure 3. Expansion and Viability of Human Memory B Cells Cultured with ImmunoCult™-ACF Human B Cell Expansion Supplement
Memory B cells were isolated from human PBMCs (leukopak) using EasySep™ Human Memory B Cell Isolation Kit and were seeded at 0.5 x 10^5 cells/well in 48-well tissue culture plates and cultured with the ImmunoCult™ Human B Cell Expansion Kit. The cells were passaged every 2 - 4 days and the fold expansion of viable cells (A) and cell viability (B) were calculated at each timepoint. Data represent the mean ± 1 SD of triplicate cultures for the same donor.
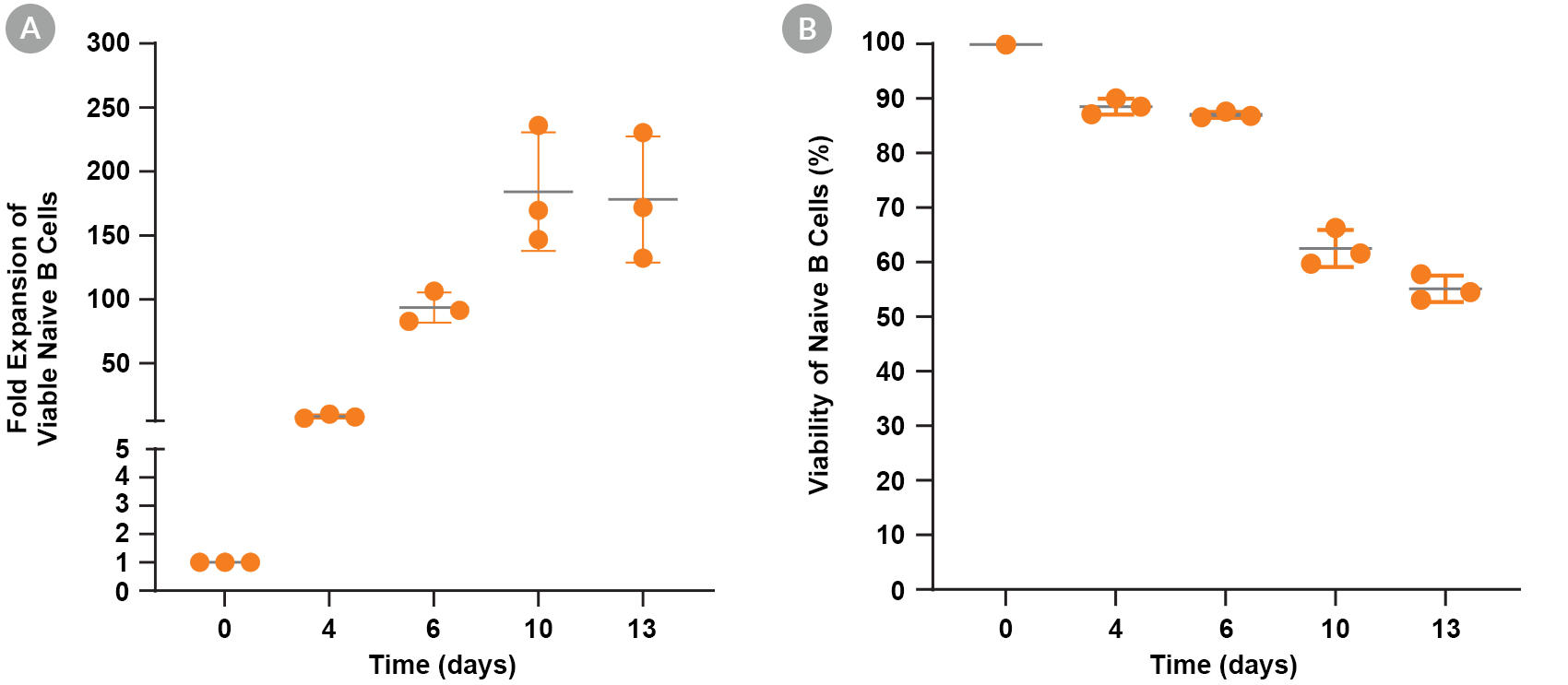
Figure 4. Expansion and Viability of Human Naive B Cells Cultured with ImmunoCult™-ACF Human B Cell Expansion Supplement
Naïve B cells were isolated from human PBMCs (leukopak) using EasySep™ Human Memory B Cell Isolation Kit and were seeded at 0.5 x 10^5 cells/well in 48-well tissue culture plates and cultured with the ImmunoCult™ Human B Cell Expansion Kit. The cells were passaged every 2 - 4 days and the fold expansion of viable cells (A) and cell viability (B) were calculated at each timepoint. Data represent the mean ± 1 SD of triplicate cultures for the same donor.
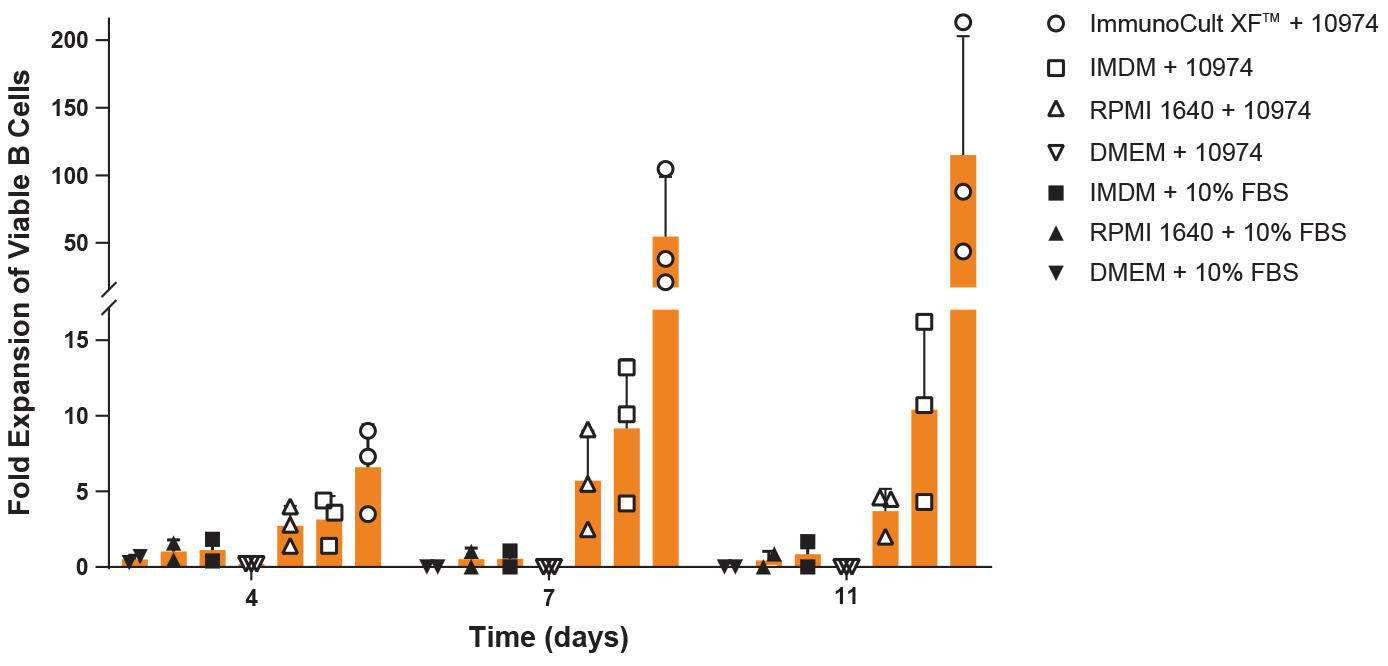
Figure 5. Expansion of Human Pan-B Cells Cultured with Various Base Media Supplemented with ImmunoCult™-ACF Human B Cell Expansion Supplement or 10% FBS
Pan-B cells were isolated from human PBMCs (Leukopak) using EasySep™ Human Pan-B Cell Enrichment Kit and seeded at 1 x 10^5 cells/well in 24-well tissue culture plates with various base media supplemented with either ImmunoCult™-ACF Human B Cell Expansion Supplement or 10% FBS. The cells were passaged every 3 - 4 days and the fold expansion of viable cells was calculated at each time point. Data represent the mean + 1SD for n = 3 donors (each culture condition was performed in triplicate).
関連製品
-

ImmunoCult-ACF Hum B Cell Expansion supplement
-
ImmunoCult-XF B Cell Base Medium
-
ImmunoCult T Cell
-
EasySep Human Pan-B Cell Enrichment Kit
-
EasySep Human CD19 Positive Selection Kit II
-
EasySep Human Memory B Cell Isolation Kit
-

EasySep Human B Cell Isolation Kit
-
EasySep Direct Human B cell Isolation kit
-
RosetteSep Human B Cell Enrichment Cocktail
-

ヒト正常末梢血細胞